Dishwasher Soap Changes Red Cabbage Juice to What Color?
Dishwasher soap changes red cabbage juice to a green color. Red cabbage juice is a natural pH indicator. In neutral conditions, it remains purple.
When it comes in contact with an acid, it turns red, and when it encounters a base or alkaline substance like dishwasher soap, it turns greenish-blue.
For example, if you add lemon juice (acidic) to red cabbage juice, it will turn into a reddish color. But if you add dishwasher soap (alkaline), it will turn into a greenish-blue hue.
The color-changing property of red cabbage juice has broad applications, from simple kitchen science experiments to complex chemical studies, making it a versatile tool in scientific exploration.
Key Takeaway
The Science Behind the Color Change
The color change of red cabbage juice when exposed to dishwasher soap is a result of the interaction between the soap’s chemical compounds and the anthocyanins in the cabbage.
- Anthocyanins are water-soluble pigments found in plants, responsible for the red, purple, and blue colors in various fruits, vegetables, and flowers.
- When mixed with a substance of a different pH, such as dishwasher soap, the anthocyanins undergo a chemical reaction, causing a shift in color.
- This phenomenon is due to a process called ‘acid-base reaction,’ where the anthocyanins act as a natural pH indicator.
The innovation lies in understanding this natural chemical reaction and its potential applications in creating eco-friendly pH indicators for various industries, such as education, food, and environmental monitoring.
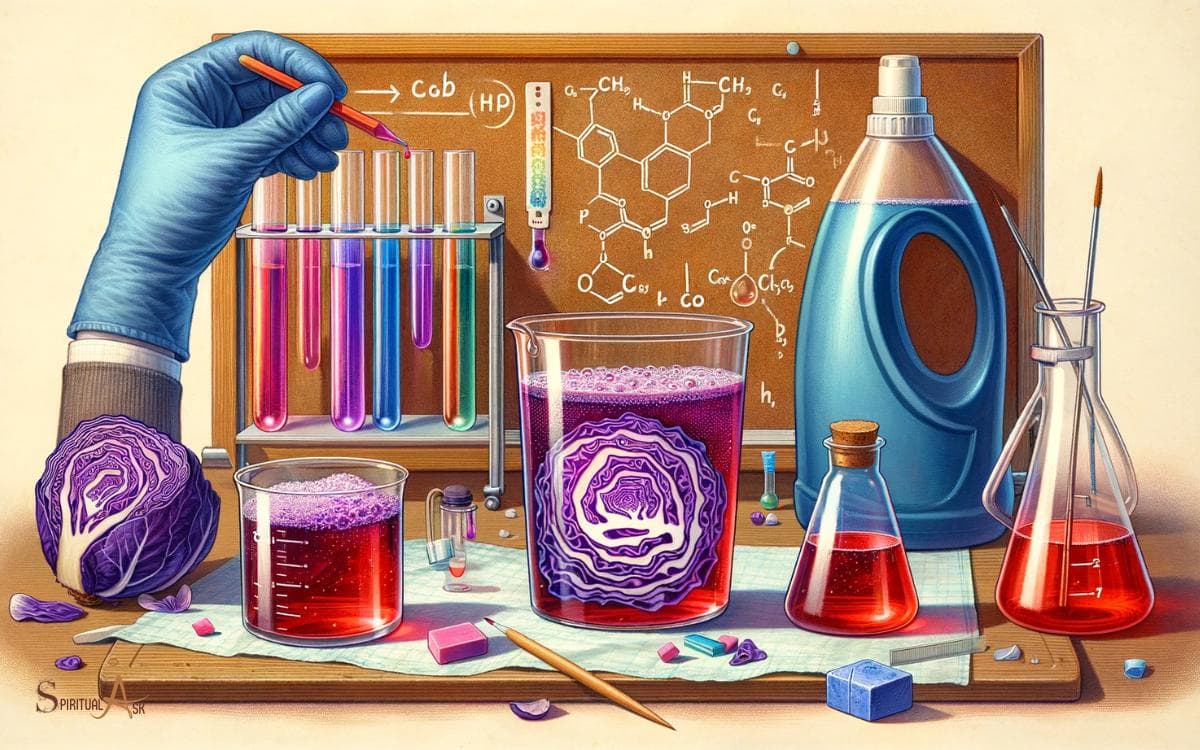
Experimenting With Red Cabbage Juice and Dishwasher Soap
- Prepare red cabbage juice using a blender and water.
- Divide the juice into separate containers for different soap concentrations.
- Add varying amounts of dishwasher soap to each container.
- Observe and record the color changes over time.
Experimenting with red cabbage juice and dishwasher soap offers an innovative way to understand the chemical reactions that occur.
By carefully documenting the color changes and comparing them to different soap concentrations, we can gain insight into the underlying chemical processes.
This experimental approach provides a hands-on way to explore the interaction between red cabbage juice and dishwasher soap, leading us to a deeper understanding of the phenomenon.
Understanding the Chemical Reaction
To comprehend the chemical reaction between red cabbage juice and dishwasher soap, it is imperative to analyze the observed color changes and correlate them with the varying concentrations of soap used in the experimentation.
- The color change is a result of a chemical reaction between the anthocyanin pigments in the red cabbage juice and the alkaline substances present in the dishwasher soap.
- When the soap is added to the red cabbage juice, the pH of the solution changes, causing the anthocyanin pigments to undergo a structural transformation, leading to the observed color alteration.
- Understanding the chemical reaction at a molecular level is crucial for unlocking the full potential of this phenomenon and exploring its practical applications.
This comprehension paves the way for further investigation into factors affecting the color transformation, such as pH levels, temperature, and the types of chemicals present in the soap.
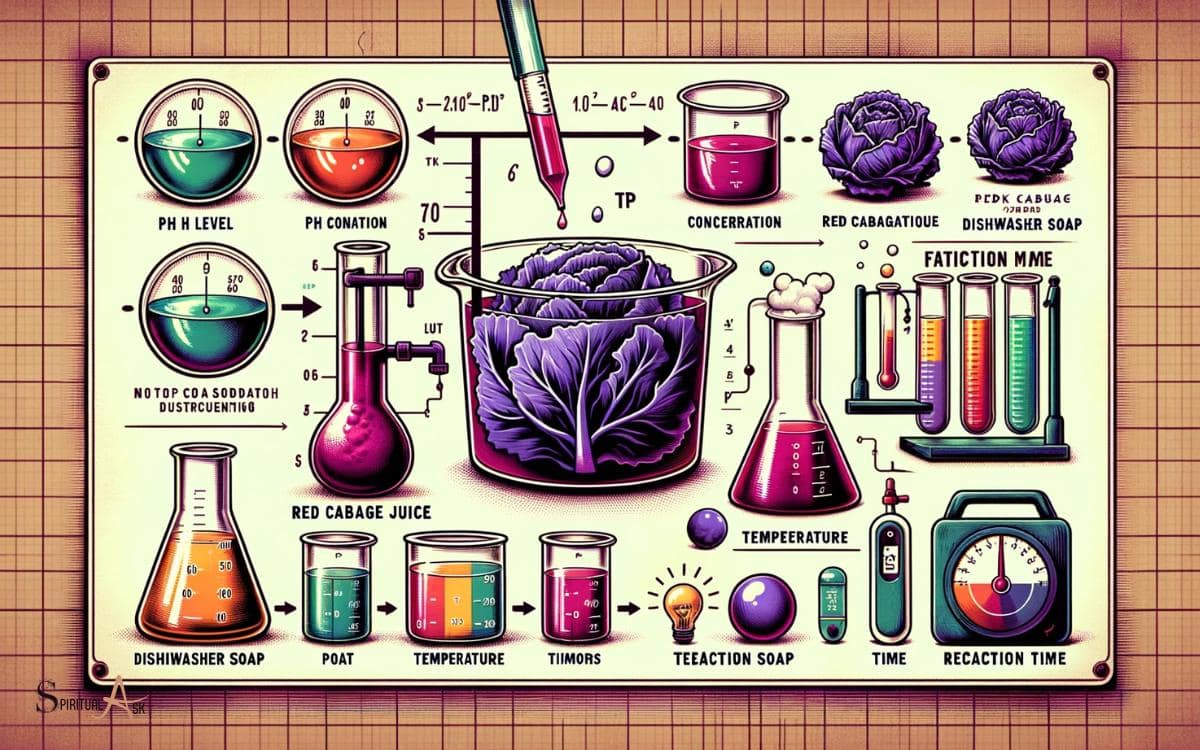
Factors Affecting the Color Transformation
Factors affecting the color transformation in the red cabbage juice due to dishwasher soap include pH levels, temperature, and the composition of the soap.
- pH Levels: The acidity or alkalinity of the solution can significantly impact the color change.
- Temperature: The rate of the chemical reaction between the soap and red cabbage juice is influenced by temperature.
- Composition of the Soap: Different ingredients in dishwasher soap can lead to variations in the color transformation.
- Presence of Catalysts or Inhibitors: Certain compounds in the dishwasher soap may act as catalysts or inhibitors, affecting the color change process.
Understanding these factors is crucial for controlling and manipulating the color transformation effectively.
This knowledge can be utilized to explore innovative applications and implications of this phenomenon in various fields.
Applications and Implications
Understanding the applications and implications of the color transformation in red cabbage juice due to dishwasher soap is essential for leveraging this phenomenon in diverse fields.
- This unique color change can be harnessed in educational settings to demonstrate chemical reactions, making science more engaging for students.
- In the field of environmental monitoring, this transformation could be utilized as an indicator for the presence of certain chemicals, aiding in pollution detection.
- Moreover, in the development of new cleaning products, this natural color-changing property could be incorporated as a visual signal for the completion of cleaning processes.
- Additionally, in the food industry, this phenomenon could be employed for quality control purposes, ensuring the effectiveness of cleaning agents in dishwashing.
Understanding and applying this color transformation could lead to innovative solutions in various industries.
Conclusion
The transformation of red cabbage juice color by dishwasher soap is a fascinating chemical reaction. By experimenting with this reaction, we gain insight into the underlying scientific principles at play.
Understanding the factors that influence this color change can have practical applications in various fields.
This research has implications for the development of innovative solutions in chemistry and beyond.